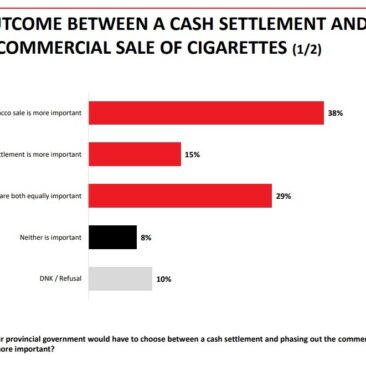
The FDA’s ground rules for IQOS may have lessons for Canada
Date:
Last month, Philip Morris began selling the heated tobacco IQOS devices and sticks in the United States. This is almost 3 years after they were marketed in Canada. The reason for the delay was that the US Food and Drug Administration would not permit the sale of this or any other new tobacco product until...